Zinc cyanide
It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.
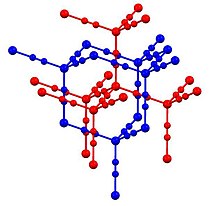
In Zn(CN)2, zinc adopts the tetrahedral coordination environment, all linked by bridging cyanide ligands.
[4] It shows one of the largest negative coefficients of thermal expansion (exceeding the previous record holder, zirconium tungstate).
The solid dissolves in, or more precisely, is degraded by, aqueous solutions of basic ligands such as hydroxide, ammonia, and additional cyanide to give anionic complexes.
[6] Zn(CN)2 is used to introduce the formyl group in to aromatic compounds in the Gatterman reaction where it serves a convenient, safer, and non-gaseous alternative to HCN.