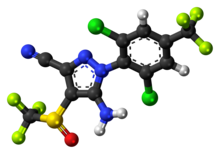
Fipronil
[6] Fipronil (IUPAC name 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-(trifluoromethylsulfinyl)pyrazole-3-carbonitrile[1]) is a white, solid powder with a moldy odor.
[9] It is now being used by the Department of Conservation to attempt local eradication of wasps,[10][11][12] and is being recommended for control of the invasive Argentine ant.
[16] Symptoms of acute toxicity via ingestion includes sweating, nausea, vomiting, headache, abdominal pain, dizziness, agitation, weakness, and tonic-clonic seizures.
The United States Environmental Protection Agency has classified fipronil as a group C (possible human) carcinogen based on an increase in thyroid follicular cell tumors in both sexes of the rat.
Commercial pet groomers and veterinary physicians were considered to be at risk from chronic exposure via inhalation and dermal absorption during the application of the spray, assuming they may have to treat up to 20 large dogs per day.
[19] In 2021, the US EPA put fipronil on the Draft Fifth Contaminant Candidate List (CCL 5) which can lead to future regulation under the Safe Drinking Water Act.
[21] Fipronil is highly toxic to crustaceans, insects (including bees and termites) and zooplankton, as well as rabbits, the fringe-toed lizard, and certain groups of gallinaceous birds.
Nontarget effects on some insects (predatory and detritivorous beetles, some parasitic wasps and bees) were also found in field trials of fipronil for desert locust control in Mauritania, and very low doses (0.6-2.0 g a.i./ha) used against grasshoppers in Niger caused impacts on nontarget insects comparable to those found with other insecticides used in grasshopper control.
[25][26] In May 2003, the French Directorate-General of Food at the Ministry of Agriculture determined that a case of mass bee mortality observed in southern France was related to acute fipronil toxicity.
In February 2003, the ministry decided to temporarily suspend the sale of BASF crop protection products containing fipronil in France.
[citation needed] Notable results from wildlife studies include: Fipronil is one of the main chemical causes blamed for the spread of colony collapse disorder among bees.
[citation needed] It has been found by the Minutes-Association for Technical Coordination Fund in France that even at very low nonlethal doses for bees, the pesticide still impairs their ability to locate their hive, resulting in large numbers of forager bees lost with every pollen-finding expedition.
[30] The functional basis for this toxic effect is now understood: the synergy between fipronil and the pathogenic fungus induces changes in male bee physiology leading to infertility.
Chicken eggs were found to contain fipronil and distributed to 15 European Union countries, Switzerland, and Hong Kong.