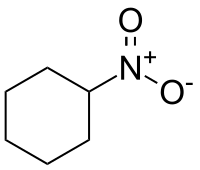
Nitrocyclohexane
It is a colorless liquid, but degraded samples appear pale yellow.
[1] It is prepared by reaction of nitrogen dioxide with cyclohexane, the so-called Nixian process.
[1] Cyclohexane is a convenient substrate because all twelve C-H bonds are equivalent, so mononitration does not give isomers (unlike the case of n-hexane).
[2] Nitrocyclohexane is highly flammable and a strong oxidizing agent.
[3] It is listed as an extremely hazardous substance by the Emergency Planning and Community Right-to-Know Act, and the NOAA warns that it can be explosive.