Glycine
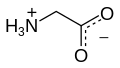
Glycine (symbol Gly or G;[6] /ˈɡlaɪsiːn/ ⓘ)[7] is an amino acid that has a single hydrogen atom as its side chain.
[8] Glycine is integral to the formation of alpha-helices in secondary protein structure due to the "flexibility" caused by such a small R group.
Glycine is also an inhibitory neurotransmitter[9] – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.
[12] Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid.
[13] He originally called it "sugar of gelatin",[14][15] but French chemist Jean-Baptiste Boussingault showed in 1838 that it contained nitrogen.
[19][20] The name comes from the Greek word γλυκύς "sweet tasting"[21] (which is also related to the prefixes glyco- and gluco-, as in glycoprotein and glucose).
[22] Although glycine can be isolated from hydrolyzed proteins, this route is not used for industrial production, as it can be manufactured more conveniently by chemical synthesis.
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate.
In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:[36] In E. coli, antibiotics that target folate depletes the supply of active tetrahydrofolates, halting glycine biosynthesis as a consequence.
This conversion is readily reversible:[36] In addition to being synthesized from serine, glycine can also be derived from threonine, choline or hydroxyproline via inter-organ metabolism of the liver and kidneys.
[8] In higher eukaryotes, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase.
In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutamatergic receptors which are excitatory.
[45] The human body rapidly clears sodium benzoate by combining it with glycine to form hippuric acid which is then excreted.
If purity greater than the USP standard is needed, for example for intravenous injections, a more expensive pharmaceutical grade glycine can be used.
[56] Glycine is also used to remove protein-labeling antibodies from Western blot membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel.
[58] The discovery of glycine in outer space bolstered the hypothesis of so-called soft-panspermia, which claims that the "building blocks" of life are widespread throughout the universe.
[62][63][64][65] For example, low complexity regions (in proteins), that may resemble the proto-peptides of the early genetic code are highly enriched in glycine.