Fluorodeoxyuridylate
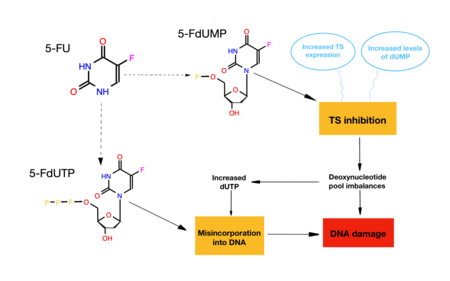
By inhibiting the deoxynucleotide biosynthesis, FdUMP stops the rapidly proliferation of fast-growing tumors, and it is therefore widely used as a cancer treatment.
Fluorouracil (5-FU) performs as a substrate during part of the catalytic cycle, and it is only during the synthesis of thymine from uridine, when it is combined with other molecules to form 5-FdUMP to produce an irreversible inhibition of the thymidylate synthase functions.
It penetrates the cell through the same facilitated transport mechanism as the uracil, due to the analogy between the two molecules (similar shape and size).
The reaction begins when a cysteine residue present at the active enzyme site attacks the pyrimidine in position 2.
After the formation of the complex, the drug loses its activity, so it is called a suicide inhibitor, as it does its function and remains inactivated due to the covalent bonds formed.
[5] But it has been shown that the conjugation of FdUMP with folic acid (FA) by a phosphodiester bonding shows improved cytotoxicity to both human and 5-FU-resistant colorectal tumor cells.