Pemetrexed
Pemetrexed, sold under the brand name Alimta among others, is a chemotherapy medication for the treatment of pleural mesothelioma and non-small cell lung cancer (NSCLC).
[6][7] In February 2004, the U.S. Food and Drug Administration (FDA) approved pemetrexed for treatment of malignant pleural mesothelioma, a type of tumor of the mesothelium, the thin layer of tissue that covers many of the internal organs, in combination with cisplatin[8] for patients whose disease is either unresectable or who are not otherwise candidates for curative surgery.
[9] In September 2008, the FDA granted approval as a first-line treatment, in combination with cisplatin, against locally advanced and metastatic non-small cell lung cancer (NSCLC) in patients with non-squamous histology.
[12][13] However, the relative efficacy or toxicity of pemetrexed-cisplatin versus pemetrexed-carboplatin has not been established beyond what is generally thought about cisplatin or carboplatin doublet drug therapy.
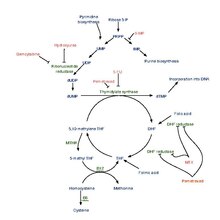
It works by inhibiting three enzymes used in purine and pyrimidine synthesis—thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase[17][18] (GARFT).