Fluxional molecule
Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening (beyond that dictated by the Heisenberg uncertainty principle) due to chemical exchange.
[2] In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods.
Prior to the advent of DNMR, kinetics of reactions were measured on non-equilibrium mixtures, monitoring the approach to equilibrium.
For processes that are too slow for traditional DNMR analysis, the technique spin saturation transfer (SST, also called EXSY for exchange spectroscopy) is applicable.
Application of the equation for coalescence of two signals separated by 10 cm−1 gives the following result:[7] Clearly, processes that induce line-broadening on the IR time-scale must be much more rapid than the cases that exchange on the NMR time scale.
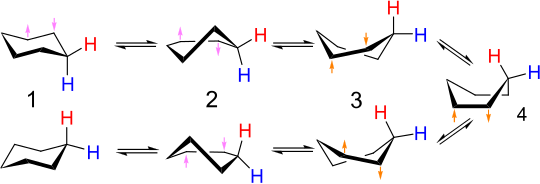
The interconversion of equivalent chair conformers of cyclohexane (and many other cyclic compounds) is called ring flipping.
Fluorine-19 NMR spectroscopy, even at temperatures as low as −100 °C, fails to distinguish the axial from the equatorial fluorine environments.
The apparent equivalency arises from the low barrier for pseudorotation via the Berry mechanism, by which the axial and equatorial fluorine atoms rapidly exchange positions.
For some compounds, dynamics occur via dissociation of a ligand, giving a pentacoordinate intermediate, which is subject to the mechanisms discussed above.