Sulfur tetrafluoride
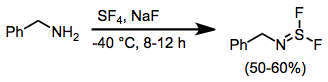
Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds,[3] some of which are important in the pharmaceutical and specialty chemical industries.
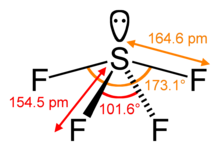
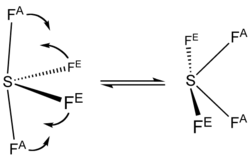
One of the three equatorial positions is occupied by a nonbonding lone pair of electrons.
The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation.
[4] At the laboratory scale, sulfur tetrafluoride is prepared from elemental sulfur and cobaltic fluoride[5] SF4 is industrially produced by the reaction of SCl2 and NaF with acetonitrile as a catalyst[6] At higher temperatures (e.g. 225–450 °C), the solvent is superfluous.
[13] Hydrolysis of SF4 gives sulfur dioxide:[14] This reaction proceeds via the intermediacy of thionyl fluoride, which usually does not interfere with the use of SF4 as a reagent.