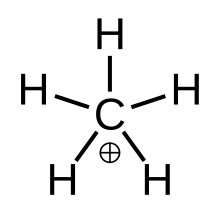
Methanium
Methanium can be produced in the laboratory as a rarefied gas or as a dilute species in superacids.
It was prepared for the first time in 1950 and published in 1952 by Victor Talrose and his assistant Anna Konstantinovna Lyubimova.
Fluxional methanium can be visualised as a CH+3 carbenium ion with a molecule of hydrogen interacting with the empty orbital in a 3-center-2-electron bond.
[5][6] Infrared spectroscopy has been used to obtain information about the different conformations of the methanium ion.
At low pressures (around 1 mmHg) and ambient temperatures, methanium is unreactive towards neutral methane.