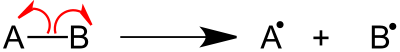
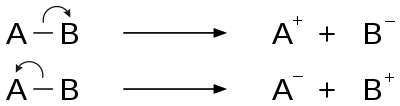
Fragmentation (mass spectrometry)
These reactions are well documented over the decades and fragmentation patterns are useful to determine the molar weight and structural information of unknown molecules.
[1][2] Fragmentation that occurs in tandem mass spectrometry experiments has been a recent focus of research, because this data helps facilitate the identification of molecules.
Relative bond energy and the ability to undergo favorable cyclic transition states affect the fragmentation process.
[2] Sigma bond cleavage is most commonly observed in molecules that can produce stable cations, such as saturated alkanes or secondary and tertiary carbocations.
This is commonly observed in alcohols, ethers, ketones, esters, amines, alkenes, and aromatic compounds with a carbon attached to ring.
This occurs in the radical cations with unsaturated functional groups, like ketones, aldehydes, carboxylic acids, esters, amides, olefins, phenylalkanes.
During this reaction, γ-hydrogen will transfer to the functional group at first and then subsequent α, β-bond cleavage of the intermediate will take place.