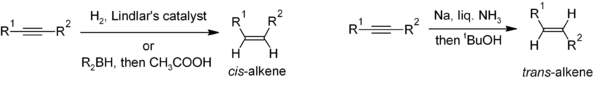Alkene
[2][3][4] Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula CnH2n with n being a >1 natural number (which is two hydrogens less than the corresponding alkane).
The simplest alkene, ethylene (C2H4) (or "ethene" in the IUPAC nomenclature) is the organic compound produced on the largest scale industrially.
Acyclic alkene structural isomers with only one double bond follow:[6] Many of these molecules exhibit cis–trans isomerism.
Rotation about the carbon–carbon double bond is restricted because it incurs an energetic cost to break the alignment of the p orbitals on the two carbon atoms.
Consequently cis or trans isomers interconvert so slowly that they can be freely handled at ambient conditions without isomerization.
The angle may vary because of steric strain introduced by nonbonded interactions between functional groups attached to the carbon atoms of the double bond.
[10] In organic chemistry,the prefixes cis- and trans- are used to describe the positions of functional groups attached to carbon atoms joined by a double bond.
E- and Z- configuration can be used instead in a more general case where all four functional groups attached to carbon atoms in a double bond are different.
The physical state depends on molecular mass: like the corresponding saturated hydrocarbons, the simplest alkenes (ethylene, propylene, and butene) are gases at room temperature.
Strained alkenes, in particular, like norbornene and trans-cyclooctene are known to have strong, unpleasant odors, a fact consistent with the stronger π complexes they form with metal ions including copper.
[15] In their 13C NMR spectra of alkenes, double bonds also deshield the carbons, making them have low field shift.
Alkenes serve as a feedstock for the petrochemical industry because they can participate in a wide variety of reactions, prominently polymerization and alkylation.
The equation of bromination of ethylene to form ethane is: Unlike hydrogenation, these halogenation reactions do not require catalysts.
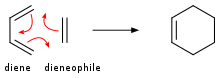
The reaction equation of HBr addition to ethylene is: Alkenes add to dienes to give cyclohexenes.
Often the reaction procedure includes a mild reductant, such as dimethylsulfide (SMe2): When treated with a hot concentrated, acidified solution of KMnO4, alkenes are cleaved to form ketones and/or carboxylic acids.
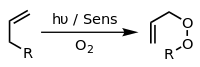
A common example is the [4+2]-cycloaddition of singlet oxygen with a diene such as cyclopentadiene to yield an endoperoxide: Terminal alkenes are precursors to polymers via processes termed polymerization.
The high reactivity of these situations is the basis for certain free radical reactions, manifested in the chemistry of drying oils.
Raw materials are mostly natural-gas condensate components (principally ethane and propane) in the US and Mideast and naphtha in Europe and Asia.
[25] Related to this is catalytic dehydrogenation, where an alkane loses hydrogen at high temperatures to produce a corresponding alkene.
Catalytic synthesis of higher α-alkenes (of the type RCH=CH2) can also be achieved by a reaction of ethylene with the organometallic compound triethylaluminium in the presence of nickel, cobalt, or platinum.
One of the principal methods for alkene synthesis in the laboratory is the elimination reaction of alkyl halides, alcohols, and similar compounds.
For unsymmetrical products, the more substituted alkenes (those with fewer hydrogens attached to the C=C) tend to predominate (see Zaitsev's rule).
A thioketone and a phosphite ester combined (the Corey-Winter olefination) or diphosphorus tetraiodide will deoxygenate glycols to alkenes.
The Cope reaction is a syn-elimination that occurs at or below 150 °C, for example:[28] The Hofmann elimination is unusual in that the less substituted (non-Zaitsev) alkene is usually the major product.
Another important class of methods for alkene synthesis involves construction of a new carbon–carbon double bond by coupling or condensation of a carbonyl compound (such as an aldehyde or ketone) to a carbanion or its equivalent.
Knoevenagel condensations are a related class of reactions that convert carbonyls into alkenes.Well-known methods are called olefinations.
Symmetrical alkenes can be prepared from a single aldehyde or ketone coupling with itself, using titanium metal reduction (the McMurry reaction).
[33] Many of the most vivid natural pigments are terpenes; e.g. lycopene (red in tomatoes), carotene (orange in carrots), and xanthophylls (yellow in egg yolk).
Although the nomenclature is not followed widely, according to IUPAC, an alkene is an acyclic hydrocarbon with just one double bond between carbon atoms.
For those cases, and for branched acyclic alkenes, the following rules apply: The position of the double bond is often inserted before the name of the chain (e.g. "2-pentene"), rather than before the suffix ("pent-2-ene").


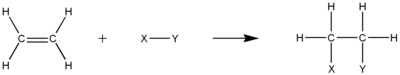

![Generation of singlet oxygen and its [4+2]-cycloaddition with cyclopentadiene](http://upload.wikimedia.org/wikipedia/commons/thumb/1/12/4%2B2_cycloaddition_cyclopentadiene_O2.svg/350px-4%2B2_cycloaddition_cyclopentadiene_O2.svg.png)