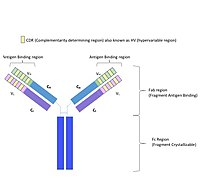
Framework region
[4] To increase its stability, the framework region has less variability in its amino acid sequences compared to the CDR.
[5][6][7] Individual residues in the framework region can be divided into two categories, depending on whether they are in direct contact with the antigen.
[4] Framework residues that do not come in contact with the antigen affect the binding indirectly by aiding in structural support for the CDR.
This enables the CDR to take on the correct orientation and position so it is exposed on the surface of the chain ready to bind to an antigen.
[8] Natural mutations in the variable region are typically due to activation-induced cytidine deaminase (AID).
This somatic hypermutation allows for immunoglobulin class switching but also results in affinity maturation of the antibody.