Fructose
It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion.
[9] Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars.
[11] Excessive consumption of sugars, including fructose, (especially from sugar-sweetened beverages) may contribute to insulin resistance, obesity, elevated LDL cholesterol and triglycerides, leading to metabolic syndrome.
The European Food Safety Authority (EFSA) stated in 2011 that fructose may be preferable over sucrose and glucose in sugar-sweetened foods and beverages because of its lower effect on postprandial blood sugar levels,[12] while also noting the potential downside that "high intakes of fructose may lead to metabolic complications such as dyslipidaemia, insulin resistance, and increased visceral adiposity".
[12][13] The UK's Scientific Advisory Committee on Nutrition in 2015 disputed the claims of fructose causing metabolic disorders, stating that "there is insufficient evidence to demonstrate that fructose intake, at levels consumed in the normal UK diet, leads to adverse health outcomes independent of any effects related to its presence as a component of total and free sugars.
"[14] The word "fructose" was coined in 1857 from the Latin for fructus (fruit) and the generic chemical suffix for sugars, -ose.
[9][15] It is also called fruit sugar and levulose or laevulose, due to its ability to rotate plane polarised light in a laevorotary fashion (anti-clockwise/to the left) when a beam is shone through it in solution.
Likewise, dextrose (an isomer of glucose) is given its name due to its ability to rotate plane polarised light in a dextrorotary fashion (clockwise/to the right).
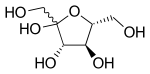
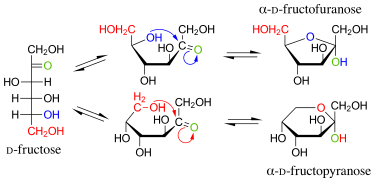
[16] Crystalline fructose adopts a cyclic six-membered structure, called β-d-fructopyranose, owing to the stability of its hemiketal and internal hydrogen-bonding.
In solution, fructose exists as an equilibrium mixture of the tautomers β-d-fructopyranose, β-d-fructofuranose, α-d-fructofuranose, α-d-fructopyranose and keto-d-fructose (the non-cyclic form).
[20] Yeast enzymes convert sugar (sucrose, glucose, and fructose, but not lactose) to ethanol and carbon dioxide.
Therefore, fructose has potential to contribute to changes in food palatability, as well as other nutritional effects, such as excessive browning, volume and tenderness reduction during cake preparation, and formation of mutagenic compounds.
This process, in the future, may become part of a low-cost, carbon-neutral system to produce replacements for petrol and diesel from plants.
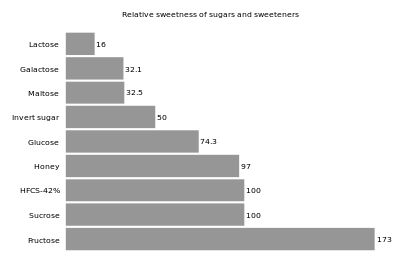
[23] The primary reason that fructose is used commercially in foods and beverages, besides its low cost, is its high relative sweetness.
[30] Fructose is quicker to absorb moisture and slower to release it to the environment than sucrose, glucose, or other nutritive sweeteners.
[29] Fructose is an excellent humectant and retains moisture for a long period of time even at low relative humidity (RH).
[24] Fructose has a greater effect on freezing point depression than disaccharides or oligosaccharides, which may protect the integrity of cell walls of fruit by reducing ice crystal formation.
The cells (enterocytes) that line children's small intestines have less affinity for fructose absorption than for glucose and sucrose.
[34] Unabsorbed fructose creates higher osmolarity in the small intestine, which draws water into the gastrointestinal tract, resulting in osmotic diarrhea.
However, with the development of HFCS, a significant shift occurred in the type of sweetener consumption in certain countries, particularly the United States.
[46] Studies show the greatest absorption rate occurs when glucose and fructose are administered in equal quantities.
However, insulin is capable of increasing the abundance and functional activity of GLUT5, fructose transporter, in skeletal muscle cells.
Increased concentrations of DHAP and glyceraldehyde 3-phosphate in the liver drive the gluconeogenic pathway toward glucose and subsequent glycogen synthesis.
[59] Carbons from dietary fructose are found in both the free fatty acid and glycerol moieties of plasma triglycerides.
High fructose consumption can lead to excess pyruvate production, causing a buildup of Krebs cycle intermediates.
[61] Triglycerides are incorporated into very-low-density lipoproteins (VLDL), which are released from the liver destined toward peripheral tissues for storage in both fat and muscle cells.
In 2022, the European Food Safety Authority stated that there is research evidence that fructose and other added free sugars may be associated with increased risk of several chronic diseases:[12][13] the risk is moderate for obesity and dyslipidemia (more than 50%), and low for non-alcoholic fatty liver disease, type 2 diabetes (from 15% to 50%) and hypertension.
EFSA further stated that clinical research did "not support a positive relationship between the intake of dietary sugars, in isocaloric exchange with other macronutrients, and any of the chronic metabolic diseases or pregnancy-related endpoints assessed" but advised "the intake of added and free sugars should be as low as possible in the context of a nutritionally adequate diet.
"[13] When fructose is consumed in excess as a sweetening agent in foods or beverages, it may be associated with increased risk of obesity, diabetes, and cardiovascular disorders that are part of metabolic syndrome.