Frustrated Lewis pair
Using FLPs to liberate H2 is metal-free, this is beneficial due to the cost and limited supply of some transition metals commonly used to activate H2 (Ni, Pd, Pt).
For example, it has been previously shown that a mixture of tricyclohexylphosphine (PCy3) and tris(pentafluorophenyl)borane reacts with H2 to give the respective phosphonium and borate ions: In this reaction, PCy3 (the Lewis base) and B(C6F5)3 (the Lewis acid) cannot form an adduct due to the steric hindrance from the bulky cyclohexyl and pentafluorophenyl groups.
The proton on the phosphorus and hydride from the borate are now ‘activated’ and can subsequently be ‘delivered’ to an organic substrate, resulting in hydrogenation.
Despite the improved ‘pre-organisational effects’, rigid intramolecular FLP frameworks are thought to have a reduced reactivity to small molecules due to a reduction in flexibility.
[7] Ethene also reacts with FLPs:[8] For acid-base pairs to behave both nucleophilically and electrophilically at the same time offers a method for the ring-opening of cyclic ethers such as THF, 2,5-dihydrofuran, coumaran, and dioxane.
It was found that increasing steric bulk of the imine substituents lead to decreased yield and ee of the amine product.
[10] Frustrated Lewis pairs of chiral alkenylboranes and phosphines are beneficial for asymmetric Piers-type hydrosilylations of 1,2-dicarbonyl compounds and alpha-keto esters, giving high yield and enantioselectivity.
Heterolytic cleavage of the Si-H bond from PhMe2SiH by the FLP catalyst forms a silylium and hydridoborate ionic complex.
[11] Metal free hydrogenation of unactivated internal alkynes to cis-alkenes is readily achieved using FLP-based catalysts.
A protodeborylation step releases the cis-alkene product, which is obtained due to the syn-hydroborylation process, and regenerating the catalyst.
The catalytic cycle has three steps: With internal alkynes, a competitive reaction occurs where the proton bound to the nitrogen can be added to the fluorobenzenes.
The metal free hydrogenation of terminal alkynes to the respective alkenes was recently achieved using a pyridone borane based system.
Furthermore, substitution of 1-methylpyrrole (which can react) with the strongly electron withdrawing tertbutyloxycarbonyl (Boc) group at the 2-position completely inhibits the reaction.
Further work by the same authors revealed that simply piperidine as the amine R group (as opposed to tetramethylpiperidine, pictured above) accelerated the rate of reaction.
Through kinetic and DFT studies the authors proposed that the C-H activation step was more facile than with larger substituents.
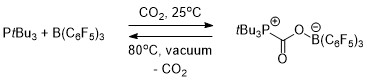
When a solution of the FLP was covered by an atmosphere of CO2 at room temperature, the FLP-CO2 compound immediately precipitated as a white solid.
[19][20] Heating the intermolecular FLP-CO2 compound in bromobenzene at 80 °C under vacuum for 5 hours caused the release of around half of the CO2 and regenerating the two constituent components of the FLP.