Phosphonium
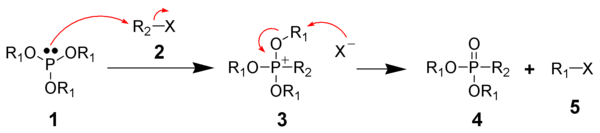
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula PR+4 (where R is a hydrogen or an alkyl, aryl, organyl or halogen group).
Salts of the parent PH+4 are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride: Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines: The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl.
[7][8] Dilute solutions dissociate according to the following equilibrium: Triphenylphosphine dichloride (Ph3PCl2) exists both as the pentacoordinate phosphorane and as the chlorotriphenylphosphonium chloride, depending on the medium.
[11] Tetrakis(hydroxymethyl)phosphonium chloride has industrial importance in the production of crease-resistant and flame-retardant finishes on cotton textiles and other cellulosic fabrics.
In 2021, Professor Doug MacFarlane and collaborators Alexandr Simonov and Bryan Suryanto of Monash University devised a method of producing green ammonia that has the potential to make Haber-Bosch plants obsolete.
While working with local company Verdant, which wanted to make bleach from saltwater by electrolysis, Suryanto discovered that a tetraalkyl phosphonium salt allowed the efficient production of ammonia at room temperature.


4 , the parent phosphonium cation.