Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4.
[4] This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides.
[6] Some commercial materials contain mineral oil to inhibit reactions with atmospheric moisture, but more commonly it is packed in moisture-proof plastic sacks.
[7] LAH violently reacts with water, including atmospheric moisture, to liberate hydrogen gas.
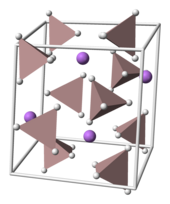
The Li+ cations are bonded to one hydrogen atom from each of the surrounding tetrahedral [AlH4]− anion creating a bipyramid arrangement.
However, it may spontaneously decompose due to the presence of catalytic impurities, though, it appears to be more stable in tetrahydrofuran (THF).
Lithium aluminium hydride (LAH) is widely used in organic chemistry as a reducing agent.
Due to its pyrophoric nature, instability, toxicity, low shelf life and handling problems associated with its reactivity, it has been replaced in the last decade, both at the small-industrial scale and for large-scale reductions by the more convenient related reagent sodium bis (2-methoxyethoxy)aluminium hydride, which exhibits similar reactivity but with higher safety, easier handling and better economics.
[33] Partial reduction of acid chlorides to give the corresponding aldehyde product cannot proceed via LAH, since the latter reduces all the way to the primary alcohol.
Instead, the milder lithium tri-tert-butoxyaluminum hydride, which reacts significantly faster with the acid chloride than with the aldehyde, must be used.
LAH also reacts with many inorganic ligands to form coordinated alumina complexes associated with lithium ions.
A substantial research effort has been devoted to accelerating the decomposition kinetics by catalytic doping and by ball milling.
Due to its high thermodynamic stability this requires temperatures in excess of 400 °C, which is not considered feasible for transportation purposes.
Accepting LiH + Al as the final product, the hydrogen storage capacity is reduced to 7.96 wt%.