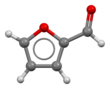
Furfural
It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust.
In addition to ethanol, acetic acid, and sugar, furfural is one of the oldest known organic chemicals available readily purified from natural precursors.
[6] Furfural was first isolated in 1821 (published in 1832) by the German chemist Johann Wolfgang Döbereiner, who produced a small sample as a byproduct of formic acid synthesis.
[7] In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of crop materials, including corn, oats, bran, and sawdust, with aqueous sulfuric acid; he also determined furfural's empirical formula (C5H4O2).
In 1870, German chemist Adolf von Baeyer speculated about the structure of the chemically similar compounds furan and 2-furoic acid.
[14] By 1887, the German chemist Willy Marckwald had inferred that some derivatives of furfural contained a furan nucleus.
The main countries producing furfural today are the Dominican Republic, South Africa and China.
The other two major commercial producers are Illovo Sugar in South Africa and Central Romana in the Dominican Republic.
Newer and more energy efficient plants have excess residue, which is or can be used for co-generation of electricity,[25][26] cattle feed, activated carbon, mulch/fertiliser, etc.
[20] Furfural is carcinogenic in lab animals and mutagenic in single cell organisms, but there is no data on human subjects.