Furan resin
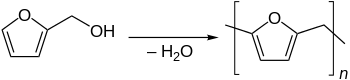
The furan monomer is typically converted to a free-flowing resin with mild acid catalysis.
In addition to homopolymers of the two starting materials, also copolymers comprising for example methanal, urea or phenol are counted as furan resins.
Furan resins based on furfuryl alcohol are produced by polycondensation under the presence of weak acids.
The polycondensation leads to various linear oligomers that differ on chainlength and linking between the furan units.
To produce a storable resin, the reaction is interrupted by the addition of sodium hydroxide.
[4] Cured furan resins are resistant to attack by strong acids, bases, and halogenated hydrocarbons.