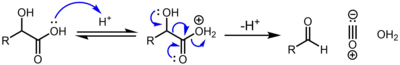
Decarbonylation
Via this reaction, formic acid is occasionally employed as a source of CO in the laboratory in lieu of cylinders of this toxic gas.
For instance, dimethylformamide ((CH3)2NC(O)H) slowly decomposes to give dimethylamine and carbon monoxide when heated to its boiling point (154 °C).
(Strictly speaking, the noncatalytic version of this reaction results in the formation of a rhodium carbonyl complex rather than free carbon monoxide.)
This reaction is generally carried out on small scale in the course of a complex natural product total synthesis, because although this reaction is very efficient at slightly elevated temperatures (e.g., 80 °C) when stoichiometric rhodium is used, catalyst turnover via extrusion of CO requires dissociation of a very stable rhodium carbonyl complex and temperatures exceeding 200 °C are required.
Some cyclic molecules containing a ketone undergo a cheletropic extrusion reaction, leaving new carbon–carbon π bonds on the remaining structure.