Glucose-6-phosphate dehydrogenase deficiency
[3] Following a specific trigger, symptoms such as yellowish skin, dark urine, shortness of breath, and feeling tired may develop.
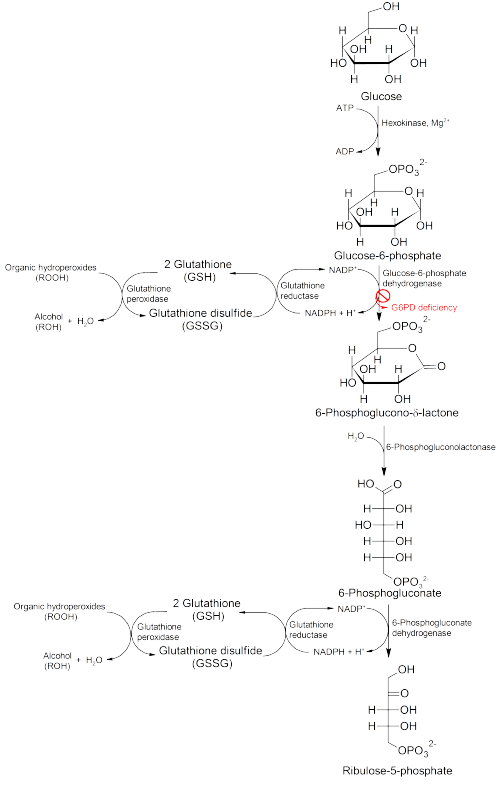
[1] Glucose-6-phosphate dehydrogenase is an enzyme that protects red blood cells, which carry oxygen from the lungs to tissues throughout the body.
[6] Red blood cell breakdown may be triggered by infections, certain medication, stress, or foods such as fava beans.
Since G6PD deficiency is not an allergy, food regulations in most countries do not require that fava beans be highlighted as an allergen on the label.
Chemical reactions involving glucose-6-phosphate dehydrogenase produce compounds that prevent reactive oxygen species from building up to toxic levels within red blood cells.
If a reduction in the amount of glucose-6-phosphate dehydrogenase or alteration of structure occurs due to the mutations of the G6PD gene, the enzyme loses its protective role and leads to the accumulation of reactive oxygen species and thus damages red blood cells.
[6] Carriers of the underlying mutation do not show any symptoms unless their red blood cells are exposed to certain triggers, which can be of four main types: Many substances are potentially harmful to people with G6PD deficiency.
Antimalarial drugs that can cause acute hemolysis in people with G6PD deficiency include primaquine, pamaquine, chloroquine, and hydroxychloroquine.
Sulfonamides (such as sulfanilamide, sulfamethoxazole, and mafenide), thiazolesulfone, methylene blue, and naphthalene should also be avoided by people with G6PD deficiency as they antagonize folate synthesis, as should certain analgesics (such as phenazopyridine and acetanilide) and a few non-sulfa antibiotics (nalidixic acid, nitrofurantoin, isoniazid, dapsone, and furazolidone).
The NADPH maintains the supply of reduced glutathione in the cells that are used to mop up free radicals that cause oxidative damage.
The role of red cells as oxygen carriers puts them at substantial risk of damage from oxidizing free radicals except for the protective effect of G6PD/NADPH/glutathione.
[citation needed] Deficiency of G6PD in the alternative pathway causes the buildup of glucose and thus there is an increase of advanced glycation endproducts (AGE).
The high prevalence of diabetes mellitus type 2 and hypertension in Afro-Caribbeans in the West could be directly related to the incidence of G6PD deficiency in those populations.
[citation needed] The diagnosis is generally suspected when patients from certain ethnic groups (see epidemiology) develop anemia, jaundice, and symptoms of hemolysis after challenges from any of the above causes, especially when there is a positive family history.
[23] When a macrophage in the spleen identifies an RBC with a Heinz body, it removes the precipitate and a small piece of the membrane, leading to characteristic "bite cells".
[citation needed] Some patients may benefit from the removal of the spleen (splenectomy),[27] as this is an important site of red cell destruction.
[citation needed] AG1, a recently discovered small molecule, has been shown to increase the activity of the G6PD enzyme in the three common variants of the deficiency.
Due to the absence of medications to treat G6PD, AG1 is a promising precursor in developing a pharmacological treatment effective for multiple G6PD enzymopathies.
[33] The Mediterranean Basin is where favism is most common, especially among Kurds, Sardinians, Cypriots, Greeks, Egyptians and some African populations, including those who have these ancestries.
[34][35][36] Favism has also been documented outside of the Mediterranean basin, in other Middle Eastern and East Asian nations like Iraq, Iran, Bulgaria and China.
This phenomenon might give G6PD deficiency carriers an evolutionary advantage by increasing their fitness in malarial endemic environments.
[39] The discovery of G6PD deficiency relied heavily upon the testing of prisoner volunteers at Illinois State Penitentiary, a type of study which today is considered unethical and cannot be performed.
Despite these results, the US military administered the drug widely during the Korean War to prevent the relapsing infection caused by Plasmodium vivax hypnozoites.
[40] After studying the mechanism through Cr51 testing, it was conclusively shown that the hemolytic effect of primaquine was due to an intrinsic defect of erythrocytes.