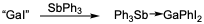Gallium monoiodide
[4] The resultant gallium monoiodide is highly air sensitive, but stable under inert atmosphere conditions for up to a year at -35 ˚C.
[4][3] When the incompletely reacted product was probed by NMR spectroscopy, it showed the presence gallium metal.
[4][7] All of the evidence from other spectroscopic methods, and power x-ray diffraction patterns, validates the assignment of [Ga0]2[Ga+][GaI4−] for the incompletely reacted gallium monoiodide variant.
[4][7] Finally, power x-ray diffraction supports that this gallium monoiodide variant matches that of characteristic Ga2I3, which is different from that of GaI2.
[4] Gallium monoiodide is used as a precursor for a variety of reactions, acting as a lewis acid and a reducing agent.
[11] The difference in reactivity between PPh3 and SbPh3, a heavy atom analogue of PPh3, can be attributed to a weaker Sb-C bond, allowing for transfer of a phenyl group from antimony to gallium.
[8][11] N-heterocyclic carbenes reacts with gallium monoiodide to form a complex with a sterically hindered isopropyl ligand.
[9] However, gallium monoiodide reacts with diazabutadienes and subsequent reduction by potassium metal to form Ga analogs of N-heterocyclic carbenes.
[8] Gallium monoiodide reacts with multidentate Lewis bases, such as bipyridine, phenyl-terpyridine, and bis(imino)pyridine ligands to form Ga(III) complexes.
More sterically hindered substituents such as tert-butyl have resulted in the formation of gallium(II) dimers, whereas reactions with alkyl or aryl substituted diazabutadienes have formed Ga(III) monomers.
[14] EPR spectroscopy has revealed that the diazabutadiene fragment is a paramagnetic monoanionic species rather than an ene-diamido dianion or a neutral ligand.
[15] Although a gallium analogue of N-heterocyclic carbenes had been synthesized previously,[16] having access to heavier analogues of N-heterocylic carbenes from a synthetically more facile gallium monoiodide route has opened new avenues in coordination chemistry, such as access to new Ga-M bonds.
Like (pentamethylcyclopentadienyl)gallium(I), cyclopentadienylgallium can also coordinate to transition metal complexes such as Cr(CO)5(cyclooctene) or Co2(CO)8 to yield CpGa–Cr(CO)5 or (thf)GaCp{Co(CO)4}2.
[25] On the other hand, cyclopentadienylgallium enables oxidative addition to Co2(CO)8 to form (thf)GaCp{Co(CO)4}2, where gallium has sigma interactions to two Co(CO)4 units.
The average Ga–Co bond length is 248.5 pm and gallium is in a formally +3 oxidation state in this new complex.
[25] Overall, straightforward synthesis of cyclopentadienylgallium from a gallium monoiodide precursor has many merits in expanding the scope of transition metal chemistry with lower valent species.
Of these products, [Ga9{Si(SiMe3)3}6]− is especially unique because Ga was found to have a very low average oxidation state (0.56) and also because this cluster has fewer R substituents than polyhedron vertices.
This highlights the versatility of the gallium monoiodide precursor in accessing a wide range of gallium-based complexes.
This is a particularly unique Co-GaI cluster due to its unusual geometry for transition metal compounds containing heavy group 13 atoms such as gallium.