Polyhedral skeletal electron pair theory
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters.
The 4n rules are reasonably accurate in predicting the structures of clusters having about 4 electrons per vertex, as is the case for many boranes and carboranes.
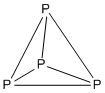
For such clusters, the structures are based on deltahedra, which are polyhedra in which every face is triangular.
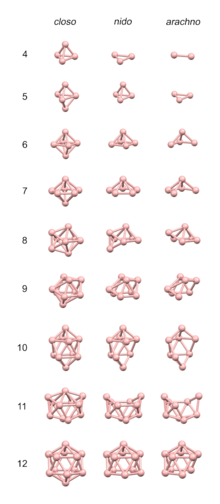
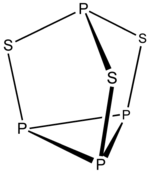
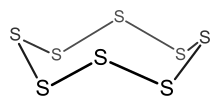
The 4n clusters are classified as closo-, nido-, arachno- or hypho-, based on whether they represent a complete (closo-) deltahedron, or a deltahedron that is missing one (nido-), two (arachno-) or three (hypho-) vertices.
However, hypho clusters are relatively uncommon due to the fact that the electron count is high enough to start to fill antibonding orbitals and destabilize the 4n structure.
A molecular orbital treatment can be used to rationalize the bonding of cluster compounds of the 4n, 5n, and 6n types.
[10] The number of vertices in the cluster determines what polyhedron the structure is based on.
To predict the structure of an arachno cluster, the closo polyhedron with n + 2 vertices is used as the starting point, and the n + 1 vertex nido complex is generated by following the rule above; a second vertex adjacent to the first is removed if the cluster is composed of mostly small atoms, a second vertex not adjacent to the first is removed if the cluster is composed mostly of large atoms.
[12][13] PSEPT also applies to metallaboranes Owing their large radii, transition metals generally form clusters that are larger than main group elements.
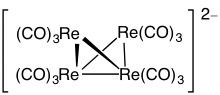

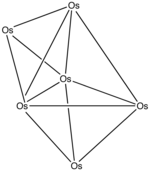
10

4
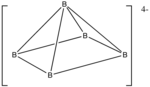
5 H 4−
5 , hydrogen atoms omitted


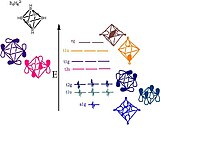
6 H 2−
6 showing the orbitals responsible for forming the cluster. Pictorial representations of the orbitals are shown; the MO sets of T and E symmetry will each have two or one additional pictorial representation, respectively, that are not shown here.