Gap junction
Gap junctions are membrane channels between adjacent cells that allow the direct exchange of cytoplasmic substances.
[14][15][16][17] A gap junction or macula communicans is different from an ephaptic coupling that involves electrical signals external to the cells.
[30] Others have presented evidence based on genetic sequencing and overall functioning in tissues, that pannexins should still be considered part of the gap junction family of proteins despite structural differences.
[83][84] Refinement of these studies suggested that gap junctions were key in the development of cell polarity[85] and the left-right symmetry in animals.
[86][87] While signaling that determines the position of body organs appears to rely on gap junctions, so does the more fundamental differentiation of cells at later stages of embryonic development.
[95] The bystander effect was later researched with regard to cells damaged by radiation or mechanical injury and in turn wound healing.
[101][102] The oral administration of gap junction blockers to reduce the symptoms of disease in remote parts of the body is slowly becoming a reality.
[103] While there has been a tendency to focus on the bystander effect in disease due to the possibility of therapeutic avenues, there is evidence that there is a more central role in normal development of tissues.
Death of some cells and their surrounding matrix may be required for a tissue to reach its final configuration; gap junctions appear essential to this process.
[104][105] There are also more complex studies that try to combine our understanding of the simultaneous roles of gap junctions in both wound healing and tissue development.
[106][107][108] Mutations in connexins have been associated with many diseases in humans, including deafness,[109] heart atrial fibrillation (standstill) and cataracts.
Tissues in this section have well known functions observed to be coordinated by gap junctions, with intercellular signaling happening in time frames of microseconds or less.
The importance is emphasized by a secondary ephaptic pathway for the signal to contract also being associated with the gap junction plaques.
This redundancy in signal transmission associated with gap junction plaques is the first to be described and involves sodium channels rather than connexins.
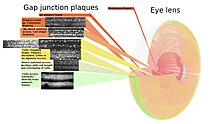
As no cells are lost from the lens interior during the life of the animal, a complete map of the gap junctions is possible.
[113] The associated figure shows how the size, shape, and frequency of gap junction plaques change with cell growth.
With growth, fiber cells are progressively isolated from more direct metabolite exchange with the aqueous humor through the capsule and lens epithelium.
Changing the fiber cells' morphology requires the movements of vesicles through the gap junction plaques at higher frequencies in this area.
[116] Moreover, mutations in the gap junction genes Cx43 and Cx56.6 cause white matter degeneration similar to that observed in Pelizaeus–Merzbacher disease and multiple sclerosis.
These proteins play crucial roles in regulating brain homeostasis through potassium buffering, intercellular communication, and nutrient transport.
[117] Connexins typically form gap junction channels that allow direct intercellular communication between astrocytes.
Studies have highlighted channel-independent functions of connexins, involving intracellular signaling, protein interactions, and cell adhesion.
[118] Specifically, Cx30 has been shown to regulate the insertion of astroglial processes into synaptic clefts, which controls the efficacy of glutamate clearance.
Consistent with all species, uterine myometrial contractions propagate from spontaneous action potentials as a result of sudden change in plasma membrane permeability.
With the eye lens, the vascular and nervous systems are absent, making reliance on hemichannels greater and their detection easier.
The close proximity of the neighboring cell membranes at the gap junction led researchers to speculate that they had a role in intercellular communication, in particular the transmission of electrical signals.
When viewed in the plane of the membrane by freeze-fracture techniques, higher-resolution distribution of connexons within the gap junction plaque is possible.
The observation was largely without explanation until vesicles were shown by Peracchia using transmission electron microscopy (TEM) thin sections to be systematically associated with gap junction plaques.
A later study using a combination of microscopy techniques confirmed the early evidence of a probable function for gap junctions in intercellular vesicle transfer.
Refined ultrastructural studies by TEM[137][138] showed protein occurred in a complementary fashion in both cells participating in a gap junction plaque.