Lipid signaling
One consequence of this is that lipid messengers cannot be stored in vesicles prior to release and so are often biosynthesized "on demand" at their intended site of action.
As such, many lipid signaling molecules cannot circulate freely in solution but, rather, exist bound to special carrier proteins in serum.
Ceramide (Cer) can be generated by the breakdown of sphingomyelin (SM) by sphingomyelinases (SMases), which are enzymes that hydrolyze the phosphocholine group from the sphingosine backbone.
Being located in the metabolic hub, ceramide leads to the formation of other sphingolipids, with the C1 hydroxyl (-OH) group as the major site of modification.
[6] However, ceramide can possibly interact with other lipids to form bigger regions called microdomains which restrict its flip-flopping abilities.
[10] On the other hand, studies in cells have shown that ceramide-inducing agents such as tumor necrosis factor-alpha α (TNFα) and palmitate induce the ceramide-dependent removal of a phosphate group (dephosphorylation) of the retinoblastoma gene product RB[11] and the enzymes, protein kinases B (AKT protein family) and C α (PKB and PKCα).
[12] Moreover, there is also sufficient evidence which implicates ceramide to the activation of the kinase suppressor of Ras (KSR),[13] PKCζ,[14][15] and cathepsin D.[16] Cathepsin D has been proposed as the main target for ceramide formed in organelles called lysosomes, making lysosomal acidic SMase enzymes one of the key players in the mitochondrial pathway of apoptosis.
Ceramide was also shown to activate PKCζ, implicating it to the inhibition of AKT, regulation of the voltage difference between the interior and exterior of the cell (membrane potential) and signaling functions that favor apoptosis.
[17] Chemotherapeutic agents such as daunorubicin and etoposide[18][19] enhance the de novo synthesis of ceramide in studies done on mammalian cells.
The same results were found for certain inducers of apoptosis particularly stimulators of receptors in a class of lymphocytes (a type of white blood cell) called B-cells.
Ceramide accumulation activates PP2A and the subsequent dephosphorylation and inactivation of AKT,[21] a crucial mediator in metabolic control and insulin signaling.
This results in a substantial decrease in insulin responsiveness (i.e. to glucose) and in the death of insulin-producing cells in the pancreas called islets of Langerhans.
[22] Inhibition of ceramide synthesis in mice via drug treatments or gene-knockout techniques prevented insulin resistance induced by fatty acids, glucocorticoids or obesity.
[23] An increase in in vitro activity of acid SMase has been observed after applying multiple stress stimuli such as ultraviolet (UV) and ionizing radiation, binding of death receptors and chemotherapeutic agents such as platinum, histone deacetylase inhibitors and paclitaxel.
Sph can also be formed in the extracellular (outer leaflet) side of the plasma membrane by the action of neutral CDase enzyme.
[28] The product sphingosine-1-phosphate (S1P) can be dephosphorylated in the ER to regenerate sphingosine by certain S1P phosphatase enzymes within cells, where the salvaged Sph is recycled to ceramide.
These targets in turn mediate the effects of Sph and its related sphingoid bases, with known roles in regulating the actin cytoskeleton, endocytosis, the cell cycle and apoptosis.
S1P is required to reach the extracellular side (outer leaflet) of the plasma membrane to interact with S1PRs and launch typical GPCR signaling pathways.
[31] The SK1-S1P pathway has been extensively studied in relation to cytokine action, with multiple functions connected to effects of TNFα and IL-1 favoring inflammation.
[43] Recent work on a sphingosine analogue, FTY270, demonstrates its ability to act as a potent compound that alters the activity of S1P receptors (agonist).
Most of the studies on S1P are used to further understand diseases such as cancer, arthritis and inflammation, diabetes, immune function and neurodegenerative disorders.
[50] In addition to their role as building blocks of biological membranes, glycosphingolipids have long attracted attention because of their supposed involvement in cell growth, differentiation, and formation of tumors.
C1P carry ionic charge at neutral pH and contain two hydrophobic chains making it relatively insoluble in aqueous environment.
As a second messenger, it is recognized by the inositol triphosphate receptor (IP3R), a Ca2+ channel in the endoplasmic reticulum (ER) membrane, which stores intracellular Ca2+.
Specifically in blood vessels, the increase in Ca2+ concentration from IP3 releases nitric oxide, which then diffuses into the smooth muscle tissue and causes relaxation.
[34] DAG remains bound to the membrane by its fatty acid "tails" where it recruits and activates both conventional and novel members of the protein kinase C family.
Elevations in either of these lipids causes analgesia and anti-inflammation and tissue protection during states of ischemia, but the precise roles played by these various endocannabinoids are still not totally known and intensive research into their function, metabolism, and regulation is ongoing.
Upon photoisomerization by a photon the cis-retinal is converted to trans-retinal causing activation of rhodopsin which ultimately leads to depolarization of the neuron thereby enabling visual perception.
See the main article on nuclear receptors This large and diverse class of steroids are biosynthesized from isoprenoids and structurally resemble cholesterol.
Retinol (vitamin A) can be metabolized to retinoic acid which activates nuclear receptors such as the RAR to control differentiation and proliferation of many types of cells during development.
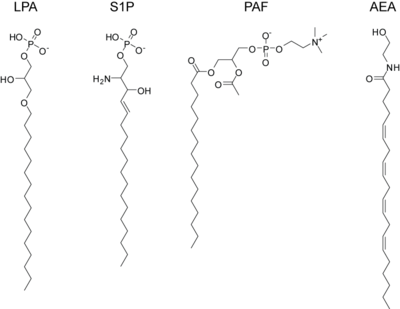
lysophosphatidic acid (LPA)
sphingosine-1-phosphate (S1P)
platelet activating factor (PAF)
anandamide or arachidonoyl ethanolamine (AEA)

