Gertrude Maud Robinson
[2] She also independently suggested the asymmetric structure of aromatic azoxy-compounds and, with her husband, postulated a mechanism for the Fischer Indole Synthesis.
[4] After moving to the University of Oxford, Gertrude Robinson began studying plant pigments and published extensively on anthocyanins with her husband.
[5] She was the first to observe that the color of a plant’s pigment was not related to the pH of its sap[2] and she pioneered work in leucoanthocyanins.
[7][note 2] They studied these pigments by comparing color distributions in immiscible solutions after reactions with alkalis or ferric chloride.
[8] The Robinsons investigated the structure of leucoanthocyanins, colorless molecules that generate anthocyanidins and are present in most plants.
[9] Leucoanthocyanins occur in more locations (wood, bark, nutshells, flowers, fruits) than normal anthocyanins.
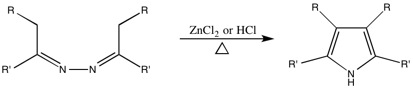
While it is unclear which Robinson the synthesis is technically named after, the paper on the topic was authored by both Gertrude and Robert.
[13][16] The Robinsons disproved many of the prevailing theories about the Fischer Indole Mechanism by showing that the reaction went unperturbed in the presence of other aromatic amines such as p-toluidine.
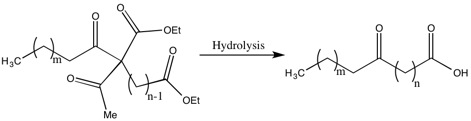
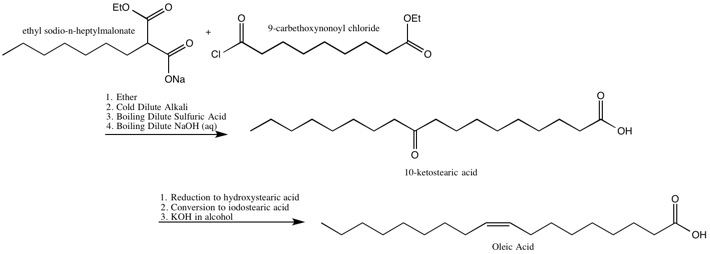
[11] One of the drawbacks of the Robinsons’ methods for the synthesis of fatty acids are the low yields due to the recoveries of a significant portion of the dialdehyde.
While she did not solve this problem, she did improve the yield and decrease the dialdehyde recovered by “the acylation of a substituted ethyl acetoacetate by the group related to the weakest possible acid”.