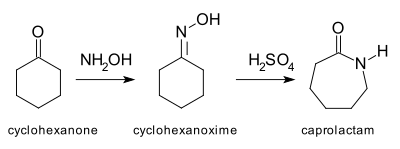
Beckmann rearrangement
[3] The Beckmann fragmentation is another reaction that often competes with the rearrangement, though careful selection of promoting reagent and solvent conditions can favor the formation of one over the other, sometimes giving almost exclusively one product.
Nitrone rearrangement also occurs without stereospecificity; the regioisomer formed has the amide nitrogen substituted with the group possessing the greatest migratory aptitude.
The most common reaction mechanism of the Beckmann rearrangement consists generally of an alkyl migration anti-periplanar to the expulsion of a leaving group to form a nitrilium ion.
This is followed by solvolysis to an imidate and then tautomerization to the amide:[6] This nitrilium ion has been known to be intercepted by other nucleophiles, including the leaving group from the oxime.
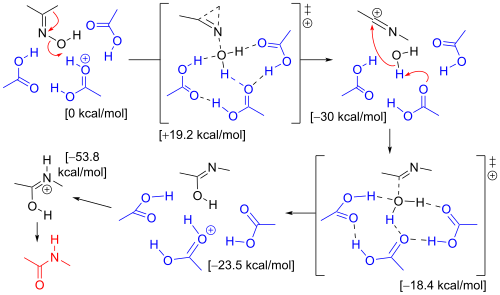
[7] The rearrangement of acetone oxime in the Beckmann solution involved three acetic acid molecules and one proton (present as an oxonium ion).
In the next step the electrophilic carbon atom in the nitrilium ion is attacked by water and a proton is donated back to acetic acid.
The same computation with a hydroxonium ion and 6 molecules of water has the same result, but when the migrating substituent is a phenyl group, the mechanism favors the formation of an intermediate three-membered π-complex.
With the cyclohexanone-oxime, the relief of ring strain results in a third reaction mechanism, leading directly to the protonated caprolactam in a single concerted step without the intermediate formation of a π-complex or σ-complex.
An industrial synthesis of paracetamol developed by Hoechst–Celanese involves the conversion of a methyl ketone to an acetanilide via a Beckmann rearrangement.