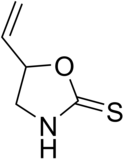
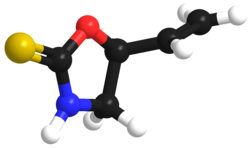
Goitrin
Goitrin is an organosulfur compound classified as a derivative of oxazolidine and as a cyclic thiocarbamate.
[1] It is found in cruciferous vegetables such as cabbage, brussels sprouts and rapeseed oil,[2] and is formed by the hydrolysis of a glucosinolate: progoitrin or 2-hydroxy-3-butenyl glucosinolate.
Hence, the oxygen in the molecule stems from the hydroxy group of the original unstable isothiocyanate.
Plants containing this specific glucosinolate (or glucosinolates such as glucobrassicin and sinalbin which liberate thiocyanate ion) have goitrogenic potential due to the goitrin and thiocyanate they contain.
However, they do not seem to alter thyroid function in humans at realistic amounts in the diet.