Grubbs catalyst
[1][2] Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents.
[5] Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.
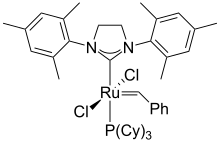
Shortly thereafter, in August 1999, Grubbs reported the second-generation catalyst, based on a saturated N-heterocyclic carbene (1,3-bis(2,4,6-trimethylphenyl)dihydroimidazole):[13] In both the saturated and unsaturated cases a phosphine ligand is replaced with an N-heterocyclic carbene (NHC), which is characteristic of all second-generation-type catalysts.
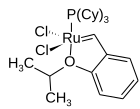
The chelating oxygen atom replaces a phosphine ligand, which in the case of the 2nd generation catalyst, gives a completely phosphine-free structure.
[20] This catalyst is used in the ring-closing metathesis reaction in water of a diene carrying an ammonium salt group making it water-soluble as well.
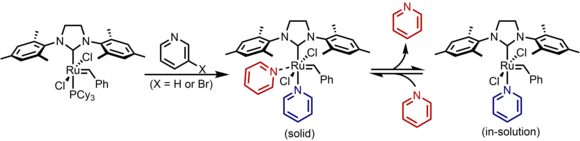
[21] Both pyridine and 3-bromopyridine are commonly used, with the bromo- version 4.8 times more labile resulting in even faster rates.
The principal application of the fast-initiating catalysts is as initiators for ring opening metathesis polymerisation (ROMP).
Large-scale commercial applications of olefin metathesis almost always employ heterogeneous catalysts or ill-defined systems based on ruthenium trichloride.