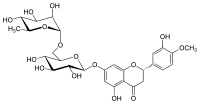
Hesperidin
Hesperidin was first isolated in 1828 by French chemist M. Lebreton from the white inner layer of citrus peels (mesocarp, albedo).
[9] As a flavanone found in the rinds of citrus fruits (such as oranges or lemons), hesperidin is under preliminary research for its possible biological properties in vivo.
One review did not find evidence that hesperidin affected blood lipid levels or hypertension.
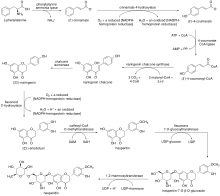
[11] The biosynthesis of hesperidin stems from the phenylpropanoid pathway, in which the natural amino acid L-phenylalanine undergoes a deamination by phenylalanine ammonia lyase to afford (E)-cinnamate.
[17] After O-methylation by caffeoyl-CoA O-methyltransferase,[18] the hesperitin product undergoes a glycosylation by flavanone 7-O-glucosyltransferase to afford hesperitin-7-O-β-D-glucoside.