High-temperature electrolysis
High-temperature electrolysis (also HTE or steam electrolysis, or HTSE) is a technology for producing hydrogen from water at high temperatures or other products, such as iron or carbon nanomaterials, as higher energy lowers needed electricity to split molecules and opens up new, potentially better electrolytes like molten salts or hydroxides.
Above 850 °C, one begins to exceed the capacity of standard chromium steels to resist corrosion,[16] and it's already no easy matter to design and implement an industrial scale chemical process to operate at such a high temperature point.
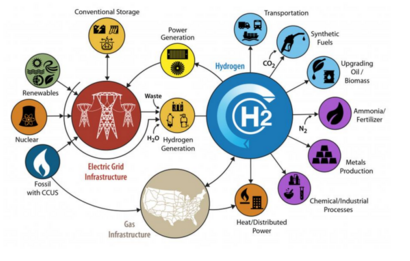
HTE is of interest as a more efficient route to the production "green" hydrogen, to be used as a carbon neutral fuel and general energy storage.
Possible supplies of cheap high-temperature heat for HTE are all nonchemical, including nuclear reactors, concentrating solar thermal collectors, and geothermal sources.
However, HTE technology suffered limitations due to: There are hundreds of thermochemical cycles known to use heat to extract hydrogen from water.
However, large-scale thermochemical production will require significant advances in materials that can withstand high-temperature, high-pressure, highly corrosive environments.
The DOE Office of Nuclear Energy has demonstration projects to test 3 nuclear facilities with high-temperature electrolysis in the United States at:[23] High temperature electrolysis with solid oxide electrolyser cells was used to produce 5.37 grams of oxygen per hour on Mars from atmospheric carbon dioxide for the Mars Oxygen ISRU Experiment in the NASA Mars 2020 Perseverance rover, using zirconia electrolysis devices.


60% efficient at 1000° C
Steam reforming of hydrocarbons to hydrogen is 70-85% efficient [ 12 ]