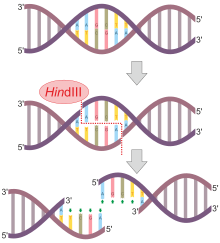
HindIII
HindIII (pronounced "Hin D Three") is a type II site-specific deoxyribonuclease restriction enzyme isolated from Haemophilus influenzae that cleaves the DNA palindromic sequence AAGCTT in the presence of the cofactor Mg2+ via hydrolysis.
[1] However, it is believed that HindIII utilizes a common mechanism of recognition and catalysis of DNA found in other type II enzymes such as EcoRI, BamHI, and BglII.
These enzymes contain the amino acid sequence motif PD-(D/E)XK to coordinate Mg2+, a cation required to cleave DNA in most type II restriction endonucleases.
Despite the fact that this residue is most likely responsible for the unwinding of DNA and coordination to water rather than direct interaction with the attacking nucleophile, its specific function is unknown.
With the aid of other van der Waals interactions, this bonding facilitates a conformational change of the DNA-enzyme complex which leads to the activation of catalytic centers.
[6] Since their discovery in the early 1970s, type II restriction enzymes have revolutionized the way scientists work with DNA, particularly in genetic engineering and molecular biology.