Carboxylate
The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegative oxygen atoms in a resonance structure.
[1]: 264–5 This delocalization of the electron means that both of the oxygen atoms are less strongly negatively charged: the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable and less basic as a result of resonance stabilization of the negative charge.
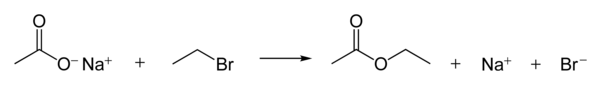
These react with alkyl halides to give derivatives:[1]: 474 Carboxylate ions are good nucleophiles.
Unlike the reduction of ester, the reduction of carboxylate is different, due to the lack of the leaving group and the relatively electron-rich carbon atom (due to the negative charge on the oxygen atoms).
With a small amount of acid, the reaction occurs with lithium aluminium hydride by changing the LAH into the Lewis acid AlH3 in the process, converting the oxyanion to 4 Al–O bonds.