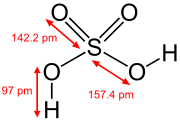
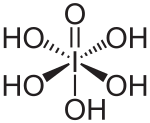
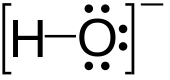
Hydroxide
It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge.
Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
The hydroxide ion is naturally produced from water by the self-ionization reaction:[2] The equilibrium constant for this reaction, defined as has a value close to 10−14 at 25 °C, so the concentration of hydroxide ions in pure water is close to 10−7 mol∙dm−3, to satisfy the equal charge constraint.
In an aqueous solution[4] the hydroxide ion is a base in the Brønsted–Lowry sense as it can accept a proton[note 4] from a Brønsted–Lowry acid to form a water molecule.
[3] In aqueous solution the hydroxide ion forms strong hydrogen bonds with water molecules.
At neutral or acid pH, the reaction is slow, but is catalyzed by the enzyme carbonic anhydrase, which effectively creates hydroxide ions at the active site.
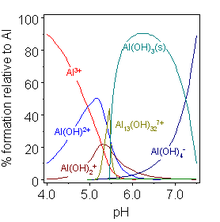
The hydroxide ion can function as a typical electron-pair donor ligand, forming such complexes as tetrahydroxoaluminate/tetrahydroxidoaluminate [Al(OH)4]−.
[6] The infrared spectra of compounds containing the OH functional group have strong absorption bands in the region centered around 3500 cm−1.
[9] Sodium hydroxide solutions, also known as lye and caustic soda, are used in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents, and as a drain cleaner.
Solutions containing the hydroxide ion are generated when a salt of a weak acid is dissolved in water.
[13] Aside from NaOH and KOH, which enjoy very large scale applications, the hydroxides of the other alkali metals also are useful.
A solution or suspension of calcium hydroxide is known as limewater and can be used to test for the weak acid carbon dioxide.
[16] In the Bayer process[20] for the production of pure aluminium oxide from bauxite minerals this equilibrium is manipulated by careful control of temperature and alkali concentration.
After removal of the insolubles, the so-called red mud, pure aluminium hydroxide is made to precipitate by reducing the temperature and adding water to the extract, which, by diluting the alkali, lowers the pH of the solution.
Perhaps the most important is the basic hydroxide AlO(OH), a polymeric material known by the names of the mineral forms boehmite or diaspore, depending on crystal structure.
When tin(II) oxide is treated with alkali the pyramidal hydroxo complex Sn(OH)−3 is formed.
The basic hydroxo complex [Pb6O(OH)6]4+ is a cluster of six lead centres with metal–metal bonds surrounding a central oxide ion.
It can also be protonated in strongly acidic conditions to give the octahedral ion [I(OH)6]+, completing the isoelectronic series, [E(OH)6]z, E = Sn, Sb, Te, I; z = −2, −1, 0, +1.
Other acids of iodine(VII) that contain hydroxide groups are known, in particular in salts such as the mesoperiodate ion that occurs in K4[I2O8(OH)2]·8H2O.
[31] As is common outside of the alkali metals, hydroxides of the elements in lower oxidation states are complicated.
One complicating feature of the hydroxides is their tendency to undergo further condensation to the oxides, a process called olation.
Some metals, e.g. V, Cr, Nb, Ta, Mo, W, tend to exist in high oxidation states.
The compound originally formulated as ZrOCl2·8H2O was found to be the chloride salt of a tetrameric cation [Zr4(OH)8(H2O)16]8+ in which there is a square of Zr4+ ions with two hydroxide groups bridging between Zr atoms on each side of the square and with four water molecules attached to each Zr atom.
[38] Copper forms hydroxyphosphate (libethenite), arsenate (olivenite), sulfate (brochantite), and nitrate compounds.
[39][42] The amphoteric hydroxide Al(OH)3 has four major crystalline forms: gibbsite (most stable), bayerite, nordstrandite, and doyleite.
[45] A similar type of hydrogen bond has been proposed for other amphoteric hydroxides, including Be(OH)2, Zn(OH)2, and Fe(OH)3.
The base should have a pKa value not less than about 4 log units smaller, or the equilibrium will lie almost completely to the left.
[49] The addition of an alcohol to an aldehyde to form a hemiacetal is an example of a reaction that can be catalyzed by the presence of hydroxide.
[52] Early methods for manufacturing soap treated triglycerides from animal fat (the ester) with lye.
The reaction medium for KOH and NaOH is usually water but with a phase-transfer catalyst the hydroxide anion can be shuttled into an organic solvent as well, for example in the generation of the reactive intermediate dichlorocarbene.





Water molecules attached to Be are omitted
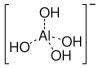
aluminate(III) ion