Immunohistochemistry
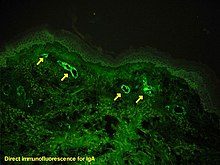
Albert Hewett Coons, Ernest Berliner, Norman Jones and Hugh J Creech was the first to develop immunofluorescence in 1941.
Immunohistochemistry is also widely used in basic research, to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of a biological tissue.
[8] Antigen retrieval is required to make the epitopes accessible for immunohistochemical staining for most formalin fixed tissue section.
Fixation of the tissue may cause formation of methylene bridges or crosslinking of amino groups, so that the epitopes no longer are available.
[9] The most common way to perform antigen retrieval is by using high-temperature heating while soaking the slides in a buffer solution.
By incubating the tissue with normal serum isolated from the species which the secondary antibody was produced, the background staining can be reduced.
Other common blocking buffers include normal serum, non-fat dry milk, BSA, or gelatin.
[5][6] Endogenous enzyme activity may also cause background staining but can be reduced if the tissue is treated with hydrogen peroxide.
Monoclonal antibodies are made by injecting the animal with the antigen of interest and then isolating an antibody-producing B cell, typically from the spleen.
While this technique utilizes only one antibody and therefore is simple and rapid, the sensitivity is lower due to little signal amplification, in contrast to indirect approaches.
[11] The indirect method, aside from its greater sensitivity, also has the advantage that only a relatively small number of standard conjugated (labeled) secondary antibodies needs to be generated.
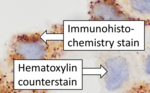
The counterstain provide contrast that helps the primary stain stand out and makes it easier to examine the tissue morphology.
[16] Endogenous biotin, reporter enzymes or primary/secondary antibody cross-reactivity are common causes of strong background staining.
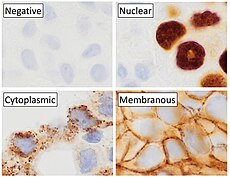
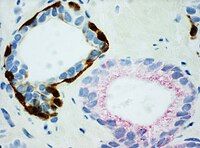
[5] [18] Immunohistochemistry is an excellent detection technique and has the tremendous advantage of being able to show exactly where a given protein is located within the tissue examined.
This has made it a widely used technique in neuroscience, enabling researchers to examine protein expression within specific brain structures.
More recently, immunohistochemical techniques have been useful in differential diagnoses of multiple forms of salivary gland, head, and neck carcinomas.
Immunohistochemistry can be used to assess which tumors are likely to respond to therapy, by detecting the presence or elevated levels of the molecular target.
[24] Many proteins shown to be highly upregulated in pathological states by immunohistochemistry are potential targets for therapies utilising monoclonal antibodies.
Among the overexpressed targets are members of the EGFR family, transmembrane proteins with an extracellular receptor domain regulating an intracellular tyrosine kinase.
There are commercially available immunohistochemical tests, Dako HercepTest,[26] Leica Biosystems Oracle[27] and Ventana Pathway.
[28] Similarly, epidermal growth factor receptor (HER-1) is overexpressed in a variety of cancers including head and neck and colon.
[29] Commercial systems to detect epidermal growth factor receptor by immunohistochemistry include the Dako pharmDx.