Histone H2B
Featuring a main globular domain and long N-terminal and C-terminal tails, H2B is involved with the structure of the nucleosomes.
[5] It plays an important role in the biology of the nucleus where it is involved in the packaging and maintaining of chromosomes,[5] regulation of transcription, and replication and repair of DNA.
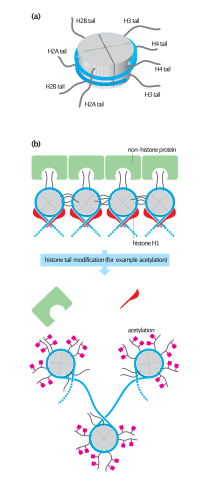
Hyperacetylation of histone tails helps DNA-binding proteins access chromatin by weakening histone-DNA and nucleosome-nucleosome interactions.
[6] Furthermore, acetylation of a specific lysine residue binds to bromine-containing domains of certain transcription and chromatin regulatory proteins.
If certain variants stopped functioning, centromeres would not form correctly, genome integrity would be lost, and the DNA damage response would be silenced.
[8] Ubiquitinase RNF20/RNF40 specifically modifies histone H2B at position K120 and this modification is need for recruitment to damaged DNA of the factors necessary for repair by the pathways of homologous recombination and non-homologous end joining.
These variants, also called isoforms, are proteins that are structurally very similar to the regular histone H2B but feature some specific variations in their amino acid sequence.
Only two to five amino acids are changed, but even these small differences can alter the isoforms higher level structure.
[1] These modifications affect the structural and functional organization of chromatin,[9] and the majority of them are found outside the globular domain of the nucleosome where amino acid residues are more accessible.
[1] Adding an acetyl group to lysine residues in one of several positions in the amino acid tail contributes to the activation of transcription.
[3] In fact, scientists consider acetylation of histone H2B's N-terminal tails, such as H2BK5ac, to be an extremely important part of regulating gene transcription.
[3] When a cell experiences metabolic stress, an AMP-activated protein kinase phosphorylates the lysine at position 36 in histone H2B of the promoter and coding regions on DNA, which helps regulate transcriptional elongation.
[2] If cells receive multiple apoptotic stimuli, caspase-3 activates the Mst1 kinase, which phosphorylates the serine at position 14 in all histone H2B proteins, which helps facilitate chromatin condensation.