Mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which can be separated by physical method.
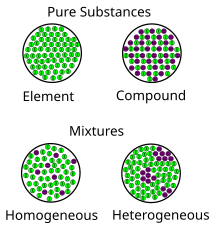
It is an impure substance made up of 2 or more elements or compounds mechanically mixed together in any proportion.
[1] A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions or colloids.
[4] Despite the fact that there are no chemical changes to its constituents, the physical properties of a mixture, such as its melting point, may differ from those of the components.
[5][6][7] All mixtures can be characterized as being separable by mechanical means (e.g. purification, distillation, electrolysis, chromatography, heat, filtration, gravitational sorting, centrifugation).
These terms are derived from the idea that a homogeneous mixture has a uniform appearance, or only one phase, because the particles are evenly distributed.
However, a heterogeneous mixture has constituent substances that are in different phases and easily distinguishable from one another.
In addition, a heterogeneous mixture may have a uniform (e.g. a colloid) or non-uniform (e.g. a pencil) composition.
Gases exhibit by far the greatest space (and, consequently, the weakest intermolecular forces) between their atoms or molecules; since intermolecular interactions are minuscule in comparison to those in liquids and solids, dilute gases very easily form solutions with one another.
Air is one such example: it can be more specifically described as a gaseous solution of oxygen and other gases dissolved in nitrogen (its major component).
(If such absence is common on macroscopic scales, the combination of the constituents is a dispersed medium, not a mixture.)
One can distinguish different characteristics of heterogeneous mixtures by the presence or absence of continuum percolation of their constituents.
[13] Making a distinction between homogeneous and heterogeneous mixtures is a matter of the scale of sampling.
On a coarse enough scale, any mixture can be said to be homogeneous, if the entire article is allowed to count as a "sample" of it.
On a fine enough scale, any mixture can be said to be heterogeneous, because a sample could be as small as a single molecule.