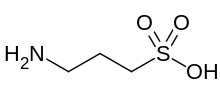
Homotaurine
[4] Homotaurine was investigated in a Phase III clinical trial as a potential treatment for Alzheimer's disease (AD) that did not show efficacy.
However, post-hoc analyses have shown positive and significant effects of homotaurine on secondary endpoints and subgroups of patients, including a reduction in hippocampal volume loss and lower decline in memory function in the overall cohort, as well as a reduction in global cognitive decline in APOE4 allele carriers, suggesting a disease-modifying effect.
[6] Homotaurine is currently in a phase 3 study with expected FDA approval as the first disease modifying drug for AD.
[4] In preclinical studies it had been found to bind to soluble amyloid beta and inhibit the formation of neurotoxic aggregates.
[15][16] In a human study homotaurine selectively and fully inhibits the formation of Aβ42 oligomers at the clinical dose, without evidence of vasogenic edema.