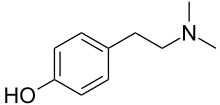
Hordenine
Hordenine is an alkaloid of the phenethylamine class that occurs naturally in a variety of plants, taking its name from one of the most common, barley (Hordeum species).
As of September 2012[update], hordenine is widely sold as an ingredient of nutritional supplements, with the claims that it is a stimulant of the central nervous system, and has the ability to promote weight loss by enhancing metabolism.
In experimental animals, given sufficiently large doses parenterally (by injection), hordenine does produce an increase in blood pressure, as well as other disturbances of the cardiovascular, respiratory, and nervous systems.
[1] Twelve years later, E. Léger independently isolated an alkaloid which he named hordenine from germinated barley (Hordeum vulgare) seeds.
[2] Ernst Späth subsequently showed that these alkaloids were identical and proposed the correct molecular structure for this substance, for which the name "hordenine" was ultimately retained.
[3] Hordenine is present in a fairly wide range of plants, notably amongst the cacti,[4] but has also been detected in some algae and fungi.
[10] In barley, hordenine levels reach a maximum within 5–11 days of germination, then slowly decrease until only traces remain after one month.
These workers concluded that hordenine production was not under significant genetic control, but much more susceptible to environmental factors such as light duration.
[1] Working with Léger's (see "Occurrence") hordenine sulfate, Camus determined minimum lethal doses for the dog, rabbit, guinea pig, and rat (see "Toxicology").
[22] In a subsequent paper, Camus reported that the intravenous (IV) administration of some hundreds of mg of hordenine sulfate to dogs or rabbits caused an increase in blood pressure and changes in the rhythm and force of contraction of the heart, noting also that the drug was not orally active.
Additional experiments on isolated tissue lead these investigators to conclude that hordenine was an indirectly acting adrenergic agent that produced its pharmacological effects by releasing stored norepinephrine (NE).
[22] From experiments aimed at identifying the toxin responsible for producing the locomotor disorder ("staggers") and rapidly lethal cardiac toxicosis ("sudden death") periodically observed in livestock feeding on the grass Phalaris aquatica, Australian researchers determined that the lowest doses of hordenine that would induce symptoms of "staggers" in sheep were 20 mg/kg IV, and 800 mg/kg orally.
[36] Hordenine has some plant growth-inhibiting properties: Liu and Lovett reported that, at a concentration of 50 ppm, it reduced the radicle length in seedlings of white mustard (Sinapis alba) by around 7%; admixture with an equal amount of gramine markedly enhanced this inhibitory effect.