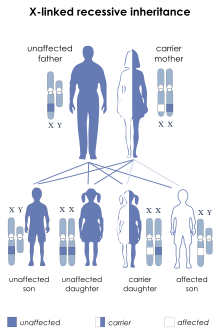
Hunter syndrome
It has traditionally been categorized as either "mild" or "severe" depending on the presence of central nervous system symptoms, but this is an oversimplification.
In milder cases, patients present similarly to children with Hurler–Scheie syndrome, and a diagnosis is usually made between the ages of 4 and 8 years.
After 18 months, children with severe MPS II may experience developmental decline and progressive loss of skills.
Progressive involvement of the finger and thumb joints results in a decreased ability to pick up small objects.
Finally, the storage of GAGs in the brain can lead to delayed development with subsequent intellectual disability and progressive loss of function.
[citation needed] The age at onset of symptoms and the presence or absence of behavioral disturbances are predictive factors of ultimate disease severity in very young patients.
The behavioral symptoms of MPS II generally precede neurodegeneration and often increase in severity until the mental handicaps become more pronounced.
[citation needed] The human body depends on a vast array of biochemical reactions to support critical functions.
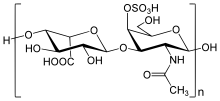
[citation needed] The biochemistry of Hunter syndrome is related to a problem in a part of the connective tissue known as the extracellular matrix, which is made up of a variety of sugars and proteins.
The rate of GAGs buildup is not the same for all people with MPS II, resulting in a wide spectrum of medical problems.
A definitive diagnosis of MPS II is made by measuring I2S activity in serum, white blood cells, or fibroblasts from skin biopsy.
[citation needed] Prenatal diagnosis is routinely available by measuring I2S enzymatic activity in amniotic fluid or in chorionic villus tissue.
Recent advances, though, have led to medications that can improve survival and well-being in people with MPS II.
Idursulfase beta, another enzyme replacement treatment, was approved in Korea by the Ministry of Food and Drug Safety.
Recent advances in enzyme replacement therapy (ERT) with idursulfase have been proven to improve many signs and symptoms of MPS II, especially if started early in the disease.
After administration, it can be transported into cells to break down GAGs, but as the medication cannot cross the blood–brain barrier, it is not expected to lead to cognitive improvement in patients with severe central nervous system symptoms.
[2] In February 2019, medical scientists working with Sangamo Therapeutics, headquartered in Richmond, California, announced the first "in body" human gene editing therapy to permanently alter DNA – in a patient with MPS II.
[9] Clinical trials by Sangamo involving gene editing using zinc finger nuclease are ongoing as of February 2019.
The cause of death is usually due to neurological complications, obstructive airway disease, and cardiac failure.
[13][14] Beginning in 2010, a phase I/II clinical trial evaluated intrathecal injections of a more concentrated dose of idursulfase than the intravenous formulation used in enzyme replacement therapy infusions, in hopes of preventing the cognitive decline associated with the severe form of the condition.
[20] On 24 July 2004, Andrew Wragg, 38, of Worthing, West Sussex, England, suffocated his 10-year-old son Jacob with a pillow, because of the boy's disabilities related to MPS II.