Hydantoin
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH.
For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.
[7] Of practical importance, hydantoins are obtained by condensation of a cyanohydrin with ammonium carbonate.
Another useful route, which follows the work of Urech, involves the condensation of amino acids with cyanates and isocyanates: The hydantoin group can be found in several medicinally important compounds.
The three major N-halogenated derivatives are dichlorodimethylhydantoin (DCDMH), bromochlorodimethylhydantoin (BCDMH), and dibromodimethylhydantoin (DBDMH).
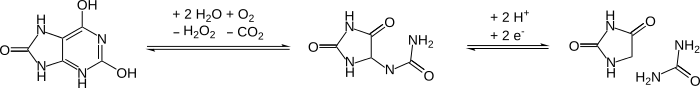
A high proportion of cytosine and thymine bases in DNA are oxidized to hydantoins over time after the death of an organism.