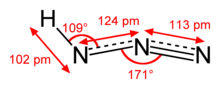
Hydrazoic acid
Undiluted hydrazoic acid is dangerously explosive[5] with a standard enthalpy of formation ΔfHo (l, 298K) = +264 kJ/mol.
[6] When dilute, the gas and aqueous solutions (<10%) can be safely prepared but should be used immediately; because of its low boiling point, hydrazoic acid is enriched upon evaporation and condensation such that dilute solutions incapable of explosion can form droplets in the headspace of the container or reactor that are capable of explosion.
The pure acid may be subsequently obtained by fractional distillation as an extremely explosive colorless liquid with an unpleasant smell.
This is one of the few reactions whose rate has been determined for specific amounts of vibrational energy in the ground electronic state, by laser photodissociation studies.
2-Furonitrile, a pharmaceutical intermediate and potential artificial sweetening agent has been prepared in good yield by treating furfural with a mixture of hydrazoic acid (HN3) and perchloric acid (HClO4) in the presence of magnesium perchlorate in the benzene solution at 35 °C.
[15][16] The all gas-phase iodine laser (AGIL) mixes gaseous hydrazoic acid with chlorine to produce excited nitrogen chloride, which is then used to cause iodine to lase; this avoids the liquid chemistry requirements of COIL lasers.