Hydride
[1] In modern usage, this is typically only used for ionic bonds, but it is sometimes (and more frequently in the past) been applied to all compounds containing covalently bound H atoms.
Almost all of the elements form binary compounds with hydrogen, the exceptions being He,[2] Ne,[3] Ar,[4] Kr,[5] Pm, Os, Ir, Rn, Fr, and Ra.
Hydrides can be discrete molecules, oligomers or polymers, ionic solids, chemisorbed monolayers,[citation needed] bulk metals (interstitial), or other materials.
Free hydride anions exist only under extreme conditions and are not invoked for homogeneous solution.
Hydrogen has a relatively low electron affinity, 72.77 kJ/mol and reacts exothermically with protons as a powerful Lewis base.
The low electron affinity of hydrogen and the strength of the H–H bond (ΔHBE = 436 kJ/mol) means that the hydride ion would also be a strong reducing agent According to the general definition, every element of the periodic table (except some noble gases) forms one or more hydrides.
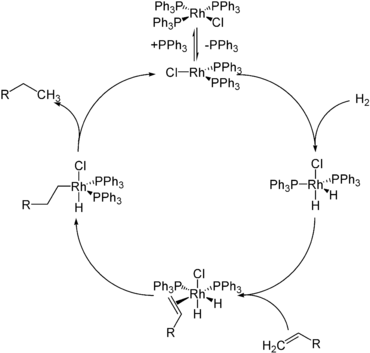
These substances have been classified into three main types according to the nature of their bonding:[6] While these divisions have not been used universally, they are still useful to understand differences in hydrides.
The divalent lanthanides such as europium and ytterbium form compounds similar to those of heavier alkaline earth metals.
This category includes hydrides that exist as discrete molecules, polymers or oligomers, and hydrogen that has been chem-adsorbed to a surface.
Particularly common are sodium borohydride (NaBH4) and lithium aluminium hydride and hindered reagents such as DIBAL.
Mechanical or thermal processing, such as bending, striking, or annealing, may cause the hydrogen to precipitate out of solution by degassing.
The first mechanism involves the adsorption of dihydrogen, succeeded by the cleaving of the H-H bond, the delocalisation of the hydrogen's electrons, and finally the diffusion of the protons into the metal lattice.
[13] Many interstitial hydrides have been developed that readily absorb and discharge hydrogen at room temperature and atmospheric pressure.
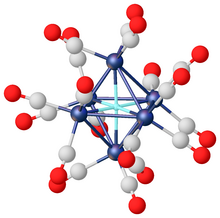
The anions potassium nonahydridorhenate [ReH9]2− and [FeH6]4− are examples from the growing collection of known molecular homoleptic metal hydrides.
Some deuterides, such as LiD, are important fusion fuels in thermonuclear weapons and useful moderators in nuclear reactors.