Chalcogenide
Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides.
The alkali metal chalcogenides often crystallize with the antifluorite structure and the alkaline earth salts in the sodium chloride motif.
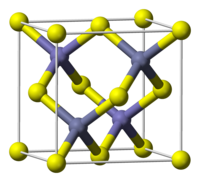
In the zinc blende structure, the sulfide atoms pack in a cubic symmetry and the Zn2+ ions occupy half of the tetrahedral holes.
Titanium disulfide was investigated in prototype cathodes for secondary batteries, exploiting its ability to reversibly undergo intercalation by lithium.
Transition metal dichalcogenides typically adopt either cadmium diiodide or molybdenum disulfide structures.
The intercalation process is accompanied by charge transfer, reducing the M(IV) centers to M(III).
The attraction between electrons and holes in 2D tungsten diselenide is 100s of times stronger than in a typical 3D semiconductor.
[8] In contrast to classical metal dichalcogenides, iron pyrite, a common mineral, is usually described as consisting of Fe2+ and the persulfido anion S22−.
[2] "Late" transition metal disulfides (Mn, Fe, Co, Ni) almost always adopt the pyrite or the related marcasite motif, in contrast to early metals (V, Ti, Mo, W) which adopt 4+ oxidation state with two chalcogenide dianions.
Amorphous MoS3 is produced by treatment of tetrathiomolybdate with acid: The mineral patrónite, which has the formula VS4, is an example of a metal tetrachalcogenide.
The structures of many main group materials are dictated by directional covalent bonding, rather than by close packing.