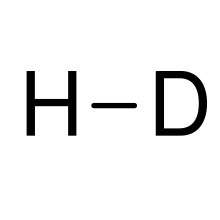Hydrogen deuteride
Its proper molecular formula is 1H2H, but for simplification, it is usually written as HD.
In the laboratory it is produced by treating sodium hydride with deuterated water:[1] Hydrogen deuteride is a minor component of naturally occurring molecular hydrogen.
It is one of the minor but noticeable components of the atmospheres of all the giant planets, with abundances from about 30 ppm to about 200 ppm.
HD has also been found in supernova remnants,[2] and other sources.
[6] The frequency of the astronomically important J = 1-0 rotational transition of HD at 2.7 THz has been measured with tunable FIR radiation with an accuracy of 150 kHz.