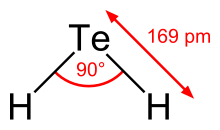
Hydrogen telluride
A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas.
Although unstable in ambient air, the gas can exist long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations.
Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.
[5] Hydrogen telluride cannot be efficiently prepared from its constituent elements, in contrast to H2Se.
[3] H2Te is an endothermic compound, degrading to the elements at room temperature: Light accelerates the decomposition.