Hydrogen chalcogenide
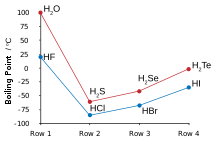
The other hydrogen chalcogenides are usually extremely toxic, and have strong unpleasant scents usually resembling rotting eggs or vegetables.
Hydrogen sulfide is a common product of decomposition in oxygen-poor environments and as such is one chemical responsible for the smell of flatulence.
Hydrogen bonding in water also results in large values of heat and entropy of vaporisation, surface tension, and viscosity.
Unlike water, however, the strong intermolecular attractions that cause the higher boiling point are van der Waals interactions, an effect of the large electron clouds of polonium.
The most important of these is hydrogen peroxide, H2O2, a pale blue, nearly colourless liquid that has a lower volatility than water and a higher density and viscosity.
[9] It is possible for two different chalcogen atoms to share a dichalcogenide, as in hydrogen thioperoxide (H2SO); more well-known compounds of similar description include sulfuric acid (H2SO4).
An alternative structural isomer of trioxidane, in which the two hydrogen atoms are attached to the central oxygen of the three-oxygen chain rather than one on each end, has been examined computationally.
H2S2 is colourless while the other polysulfanes are yellow; the colour becomes richer as n increases, as do the density, viscosity, and boiling point.
[13] However, they can easily be oxidised and are all thermally unstable, disproportionating readily to sulfur and hydrogen sulfide, a reaction for which alkali acts as a catalyst:[13] They also react with sulfite and cyanide to produce thiosulfate and thiocyanate respectively.
[13] An alternative structural isomer of the trisulfide, in which the two hydrogen atoms are attached to the central sulfur of the three-sulfur chain rather than one on each end, has been examined computationally.
[14] Thiosulfuric acid, in which two sulfur atoms branch off of the central of a linear dihydrogen trisulfide structure has been studied computationally as well.
Due to the high difference in density between deuterium and regular protium, heavy water exhibits many anomalous properties.