Hydrohalite
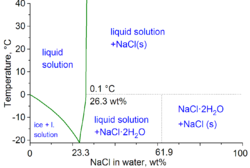
Hydrohalite is a mineral that occurs in saturated halite brines at cold temperatures (below 0.1 °C).
Hydrohalite has a high nucleation energy, and solutions will normally need to be supercooled for crystals to form.
Above this temperature, liquid water saturated with salt can exist in equilibrium with hydrohalite.
Hydrohalite has a strong positive temperature coefficient of solubility, unlike halite.
[2] Hydrohalite decomposes at 0.1°C, giving a salty brine and solid halite.