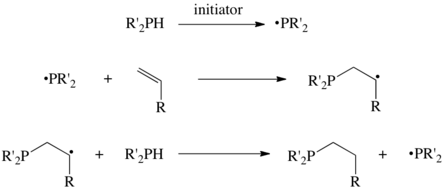
Hydrophosphination
Typically base-catalysis allows addition of Michael acceptors such as acrylonitrile to give tris(cyanoethyl)phosphine:[2] Acid catalysis is applicable to hydrophosphination with alkenes that form stable carbocations.
These alkenes include isobutylene and many analogues:[2] Bases catalyze the addition of secondary phosphines to vinyldiphenylphosphine:[3] Many hydrophosphination reactions are initiated by free-radicals.
These substrates bind to metals, and the resulting adducts insert alkenes and alkynes into the P-H bonds via diverse mechanisms.
The primary phosphine undergoes a σ-bond metathesis with the bis(trimethylsilyl)methylene ligand forming the lanthanide-phosphido complex.
[11] This system involves a cationic catalyst precursor that is stabilized by the bulky 2,4,6-tri(isopropyl)phenyl- substituent on the phosphinidene and the close ionic association of methyltris(pentafluorophenyl)borate.
The substrate, phenylphosphine, protonolyzes the Ti-C bond and after a proton shift regenerates the catalyst and releases the new phosphine.
Late transition metal hydrophosphination catalysts, i.e. those reliant on the nickel-triad and neighboring elements, generally require alkenes and alkynes with electron withdrawing substituents.
Utilizing phosphorus(V) precursors hydrophosphorylation entails the insertion of alkenes and alkynes into the P-H bonds of secondary phosphine oxides:[15] The reaction can be effected both using metal catalysts or free-radical initiators.