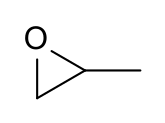
Propylene oxide
[10] The traditional route proceeds via the conversion of propene to propylene chlorohydrin according to the following simplified scheme: The mixture of 1-chloro-2-propanol and 2-chloro-1-propanol is then dehydrochlorinated.
Propylene oxide is commonly used in the preparation of biological samples for electron microscopy, to remove residual ethanol previously used for dehydration.
[20] Signs of toxicity after acute exposure include salivation, lacrimation, nasal discharge, gasping, lethargy and hypoactivity, weakness, and incoordination.
[22][23][24] Pregnant rats exposed to 500ppm of propylene oxide for less than 8 hours gave birth to litters with significant deformities and weight deficiencies.
When exposed to an open atmosphere, the vapor can accumulate in low-lying areas while spreading out over long distances and reach ignition source, causing flashback or an explosion.
[25][28] When heated, propylene oxide can rapidly self-polymerize and decompose producing other toxic gases such as carbon monoxide and various free radicals.
[25] When burning in open air however, water can transport propylene oxide outside of the fire zone which can reignite upon floating to the surface.
Additional firefighting measures should be taken to prevent propylene oxide from washing out to nearby drains and sewers contaminating the surrounding environment.
[30][25] In 2016 it was reported that propylene oxide was detected in Sagittarius B2, a cloud of gas in the Milky Way weighing three million solar masses.