Bilirubin
Bilirubin (BR) (from the Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates.
This catabolism is a necessary process in the body's clearance of waste products that arise from the destruction of aged or abnormal red blood cells.
Heme then passes through various processes of porphyrin catabolism, which varies according to the region of the body in which the breakdown occurs.
[5][6] Ultimately, bilirubin is broken down within the body, and its metabolites excreted through bile and urine; elevated levels may indicate certain diseases.
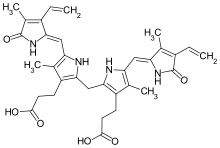
[citation needed] Some textbooks and research articles show the incorrect geometric isomer of bilirubin.
[16][17] Consistent with this, animal studies suggest that eliminating bilirubin results in endogenous oxidative stress.
[18] Bilirubin's antioxidant activity may be particularly important in the brain, where it prevents excitotoxicity and neuronal death by scavenging superoxide during N-methyl-D-aspartic acid neurotransmission.
[11] Bilirubin is then released into the plasma and transported to the liver bound by albumin, since it is insoluble in water in this state.
[11] In the liver, unconjugated bilirubin is up-taken by the hepatocytes and subsequently conjugated with glucuronic acid (via the enzyme uridine diphosphate–glucuronyl transferase).
[11][20] In parallel, a small amount of conjugated billirubin can also enter the systemic circulation and get excreted through urine.
Over time, when red blood cells need to be replenished, the hemoglobin is broken down in the spleen; it breaks down into two parts: heme group consisting of iron and bile and protein fraction.
[citation needed] Unbound bilirubin (Bf) levels can be used to predict the risk of neurodevelopmental handicaps within infants.
[28] Unconjugated hyperbilirubinemia in a newborn can lead to accumulation of bilirubin in certain brain regions (particularly the basal nuclei) with consequent irreversible damage to these areas manifesting as various neurological deficits, seizures, abnormal reflexes and eye movements.
In addition, recent studies point towards high total bilirubin levels as a cause for gallstones regardless of gender or age.
[30] Studies have also revealed that levels of serum bilirubin (SBR)[31] are inversely related to risk of certain heart diseases.
[34] In addition to this, there have been recent discoveries linking bilirubin and its ε-polylysine-bilirubin conjugate (PLL-BR), to more efficient insulin medication.
It seems that bilirubin exhibits protective properties during the islet transplantation process when drugs are delivered throughout the bloodstream.
Though most bile acid is reabsorbed in the terminal ileum to participate in enterohepatic circulation, conjugated bilirubin is not absorbed and instead passes into the colon.
[45] There, colonic bacteria deconjugate and metabolize the bilirubin into colorless urobilinogen, which can be oxidized to form urobilin and stercobilin.
[citation needed] This test is performed routinely in most medical laboratories and can be measured by a variety of methods.
Testing urine for both bilirubin and urobilinogen can help differentiate obstructive liver disease from other causes of jaundice.
If the liver's function is impaired or when biliary drainage is blocked, some of the conjugated bilirubin leaks out of the hepatocytes and appears in the urine, turning it dark amber.
[62] Relevant documentation emerged in 1827 when M. Louis Jacques Thénard examined the biliary tract of an elephant that had died at a Paris zoo.
He observed dilated bile ducts were full of yellow magma, which he isolated and found to be insoluble in water.
[62] Leopold Gmelin experimented with nitric acid in 1826 to establish the redox behavior in change from bilirubin to biliverdin, although the nomenclature did not exist at the time.
[62] The term biliverdin was coined by Jöns Jacob Berzelius in 1840, although he preferred "bilifulvin" (yellow/red) over "bilirubin" (red).
[69] Plieninger and Fischer demonstrated an enzymatic oxidative loss of the alpha-methine bridge of heme resulting in a bis-lactam structure in 1942.